The Silent Struggle: How Metabolic Fatty Liver Disease Leads to Hepatopulmonary Syndrome
Understanding Metabolic-Associated Fatty Liver Disease as a Cause of Hepatopulmonary Syndrome
Overview
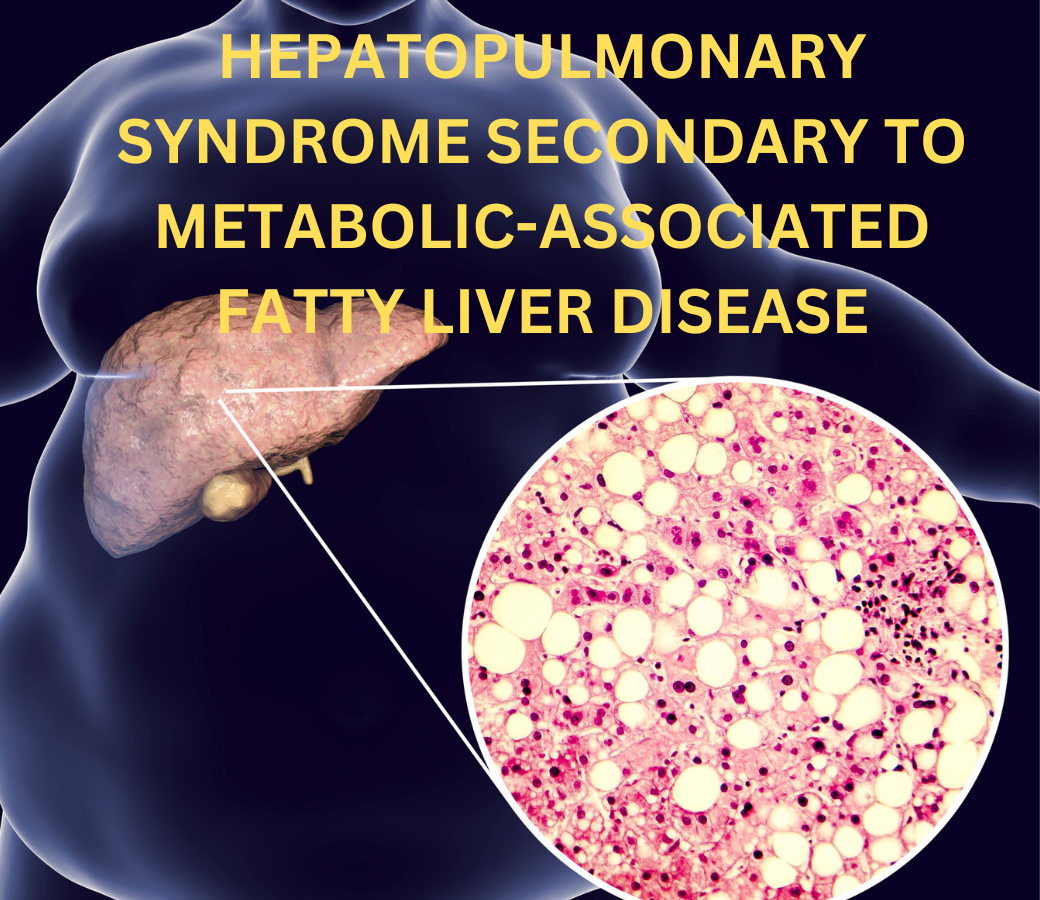
Millions of people worldwide suffer from Metabolic-Associated Fatty Liver Disease (MAFLD), which has become one of the most prevalent liver diseases. When there is little to no alcohol consumption, it is marked by the buildup of fat in the liver. Although issues related to the liver are the main consequence of MAFLD, it can also lead to extrahepatic diseases, such as the less well-known but potentially fatal Hepatopulmonary Syndrome (HPS). By examining the mechanism, clinical symptoms, diagnosis, and therapy of HPS owing to MAFLD, this article seeks to provide readers a thorough grasp of the condition.
A Synopsis of Metabolic-Associated Fatty Liver Disease (MAFLD)

MAFLD, formerly known as Non-Alcoholic Fatty Liver Disease (NAFLD), is a term used to describe a group of liver disorders that can range in severity from non-alcoholic steatohepatitis (NASH), fibrosis, and cirrhosis to simple steatosis (fat buildup). It is intimately linked to metabolic conditions that accelerate the course of the disease, including dyslipidemia, obesity, type 2 diabetes, and hypertension.
MAFLD prevalence is rising globally in tandem with rising rates of metabolic syndrome and obesity. Although the main symptom of MAFLD is liver disease, it affects more than just the liver. As the illness worsens, it may cause systemic side effects such as chronic kidney disease, heart problems, and respiratory disorders such as hepatopulmonary syndrome (HPS).
Hepatopulmonary Syndrome (HPS): What is it?
Hepatopulmonary Syndrome (HPS) is an uncommon but potentially fatal lung consequence of liver illness. The three conditions that define it are intrapulmonary vascular dilatation (IPVD), arterial hypoxemia (low blood oxygen levels), and liver disease. The cause of HPS is aberrant dilation of the pulmonary blood vessels, which impairs oxygen exchange and lowers blood oxygen levels.
Studies indicate that between 10 and 30 percent of people with cirrhosis may have HPS, while the precise prevalence of the condition varies amongst patients with liver disease. Although HPS is most frequently linked to cirrhosis, it can also occur in people with liver diseases other than cirrhosis, such as MAFLD, particularly when it is further advanced.
HPS pathophysiology in MAFLD
In the case of MAFLD, HPS development is intricate and involves several mechanisms. The primary pathological characteristic of HPS is the dilating of the pulmonary tiny blood vessels, which interferes with the regular flow of oxygen from the bloodstream to the air sacs (alveoli). This results in inadequately oxygenated blood flow via the lungs, a condition known as ventilation-perfusion mismatch.
In patients with MAFLD, a number of variables contribute to the development of HPS:
Overproduction of Nitric Oxide (NO): Nitric oxide is a powerful vasodilator that is essential for controlling blood vessel tone. Nitric oxide is produced in excess in the lungs of individuals with liver disease, especially MAFLD, which causes the pulmonary blood vessels to dilate excessively. Poor oxygenation results from the blood bypassing the alveoli as a result of this severe dilation.
Dysfunction of the Endothelium: MAFLD and HPS are characterized by dysfunction of the endothelium, which is the inner lining of blood vessels. The atypical pulmonary artery dilatation observed in HPS is partly caused by endothelial damage resulting from systemic inflammation and oxidative stress in MAFLD.
Impaired Liver Function: The liver’s capacity to detoxify dangerous substances declines as MAFLD develops into more severe types like NASH and cirrhosis. This causes poisons and inflammatory mediators to build up in the blood, which can worsen pulmonary vascular dilatation and the onset of HPS.
Angiogenesis: The process by which new blood vessels form is also linked to the pathophysiology of HPS. Vascular endothelial growth factor (VEGF) and other angiogenic factors are high in MAFLD, which encourages the creation of aberrant blood vessels in the lungs and adds to the vascular abnormalities observed in HPS.
HPS’s Clinical Symptoms in MAFLD
Numerous respiratory symptoms, some of which may be mild and easily missed in the early stages, might be present with HPS. The following are the most typical clinical signs of HPS in individuals with MAFLD:
The main sign of HPS is dyspnea, or shortness of breath, which can be worse when the patient is seated or standing erect (orthodeoxia). The distinctive positional progression of symptoms distinguishes HPS from other respiratory disorders.
The term “platypnea” describes the alleviation of dyspnea while in a supine position. This contrasts with orthopnea, which is a condition associated with heart failure in which breathing becomes more difficult when resting flat.
Cyanosis: Low blood oxygen levels can result in cyanosis, or bluish coloring of the skin and mucous membranes. It is frequently easier to see on the lips, fingertips, and toes.
Thickness of the fingertips, or “digital clubbing,” is a typical observation in HPS patients and is indicative of persistent hypoxemia.
Fatigue: Prolonged low oxygen levels can cause weariness and a decreased capacity for exercise, which can have a serious negative effect on the patient’s quality of life.
HPS diagnosis in MAFLD
A strong index of suspicion is necessary for the diagnosis of HPS, particularly in patients with advanced MAFLD who arrive with inexplicable dyspnea. To confirm the presence of intrapulmonary vascular dilatation and arterial hypoxemia, a mix of imaging techniques, specialist testing, and clinical evaluation are used in the diagnostic procedure.
Analysis of Arterial Blood Gas (ABG): ABG analysis is necessary to determine blood oxygen levels. ABG usually shows hypoxemia (a partial pressure of oxygen, or PaO2) of less than 80 mmHg in HPS patients.
The most reliable method for identifying intrapulmonary vascular dilatation is contrast-enhanced echocardiography. In order to do an echocardiogram, agitated saline is injected into a peripheral vein. One important aspect of HPS is intrapulmonary shunting, which is indicated by the presence of microbubbles in the left atrium after they have left the lungs.
PFTs, or pulmonary function tests, may reveal a mildly restrictive pattern in HPS patients. These tests, however, are primarily used to evaluate general lung function and are not specific for HPS.
Liver Function Tests and Imaging: Because liver disease is linked to liver function issues, liver function tests and imaging tests like MRIs and ultrasounds are crucial for determining the nature and severity of the underlying liver ailment.
A nuclear medicine test called the 99mTc-Macroaggregated Albumin (MAA) Lung Perfusion Scan can be performed to measure the extent of intrapulmonary shunting. A radiotracer that is typically stuck in the lung capillaries is injected during the procedure. The tracer in HPS avoids the lungs and instead finds its way to the brain or kidneys.
HPS Secondary Management to MAFLD
It is difficult to manage HPS related to MAFLD and calls for a multidisciplinary team effort including transplant specialists, pulmonologists, and hepatologists. Optimizing oxygenation, controlling symptoms, and treating the underlying liver disease are the main objectives of treatment.
Oxygen Therapy: The cornerstone of treating HPS symptoms is more oxygen. It does not cure the underlying vascular problems, but it does aid with dyspnea and oxygen saturation.
Liver transplantation: Because it can reverse the pulmonary vascular abnormalities and restore normal oxygenation, liver transplantation is the only conclusive treatment for HPS. Liver transplantation may be considered for patients with advanced HPS and MAFLD, particularly if no other treatment options are working.
Medical Therapies: As of right now, no particular medical treatments for HPS have been approved. Nonetheless, research is being done on a few experimental therapies that target the nitric oxide pathways and angiogenesis. The goals of these treatments are to increase oxygenation and decrease pulmonary vascular dilatation.
Handling MAFLD: In order to effectively manage HPS, the underlying MAFLD must be addressed. Improving liver function and delaying the course of the disease require lifestyle adjustments such as losing weight, eating differently, and engaging in regular physical activity. Medication such as lipid-lowering medicines or insulin sensitizers may be recommended in certain situations to control metabolic risk factors.
Monitoring and Follow-Up: It’s critical to regularly check in with a medical professional to track the development of MAFLD and HPS. Patients need to have their liver function, oxygen levels, and symptoms regularly evaluated.
In summary
Metabolic-Associated Fatty Liver Disease (MAFLD)-related hepatopulmonary syndrome (HPS) is a complicated and potentially fatal illness that needs to be treated with caution. Awareness and comprehension of MAFLD’s extrahepatic consequences, such as HPS, are becoming more and more crucial as the disease’s prevalence increases worldwide. For impacted patients to have better outcomes and a higher quality of life, early diagnosis, prompt management, and a comprehensive approach to managing both the liver disease and the pulmonary consequences are crucial. Although liver transplantation is now the only effective treatment for HPS, continued research into new treatments shows promise for the management of this difficult illness in the future.
 https://analytics.google.com/analytics/web/#/analysis/p405220706
Skip to content
https://analytics.google.com/analytics/web/#/analysis/p405220706
Skip to content 
4 thoughts on ““The Silent Struggle: How Metabolic Fatty Liver Disease Leads to Hepatopulmonary Syndrome””